
Mucopolysaccharidosis Market Analysis Across 7MM — Unveiling Growth Opportunities Across Different Types | DelveInsight
The mucopolysaccharidosis market is witnessing a surge in innovation, driven by cutting-edge gene therapies, enzyme replacement treatments, and novel drug developments. The market is poised for exponential growth with rising awareness, early diagnosis, and increasing investments in rare disease research.
/EIN News/ -- New York, USA, Feb. 18, 2025 (GLOBE NEWSWIRE) -- Mucopolysaccharidosis Market Analysis Across 7MM — Unveiling Growth Opportunities Across Different Types | DelveInsight
The mucopolysaccharidosis market is witnessing a surge in innovation, driven by cutting-edge gene therapies, enzyme replacement treatments, and novel drug developments. The market is poised for exponential growth with rising awareness, early diagnosis, and increasing investments in rare disease research.
Mucopolysaccharidosis (MPS) is a group of rare genetic disorders caused by the deficiency of specific lysosomal enzymes responsible for breaking down glycosaminoglycans (GAGs), formerly known as mucopolysaccharides. As a result, these complex sugars accumulate in cells, leading to progressive tissue and organ damage. MPS disorders are inherited in an autosomal recessive manner, except for MPS II (Hunter syndrome), which is X-linked.
Symptoms vary depending on the specific type of MPS but commonly include skeletal abnormalities, coarse facial features, developmental delays, organ enlargement, hearing loss, and respiratory or cardiac complications. Some forms, like MPS I (Hurler syndrome) and MPS II, also involve severe neurological decline, while others, such as MPS IV (Morquio syndrome), primarily affect the skeletal system without significant cognitive impairment.
The treatment landscape for MPS includes enzyme replacement therapy (ERT), hematopoietic stem cell transplantation (HSCT), and supportive care to manage symptoms. ERT, available for conditions like MPS I, II, and VI, helps reduce systemic complications but has a limited impact on neurological symptoms due to the blood-brain barrier. HSCT is more effective in preventing neurodegeneration, particularly when performed early, but carries significant risks.
Gene therapy is emerging as a potential long-term solution, aiming to deliver functional copies of the defective gene. Supportive care, including physical therapy, surgeries for skeletal and cardiac issues, and symptom-specific management, remains crucial for improving patients’ quality of life. Ongoing research continues to explore novel therapeutic approaches, such as intrathecal enzyme delivery and substrate reduction therapy, to address unmet medical needs in MPS.
Discover more about the mucopolysaccharidosis market in detail @ Mucopolysaccharidosis Market Report
DelveInsight has expertise in the rare disease market, and an experienced team handles the rare disease domain proficiently. DelveInsight has recently released a series of epidemiology-based market reports on different types of mucopolysaccharidosis including Mucopolysaccharidosis I, Mucopolysaccharidosis II, Mucopolysaccharidosis III, Mucopolysaccharidosis IV, and Mucopolysaccharidosis VII. These reports include a comprehensive understanding of current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted market size from 2020 to 2034 segmented into 7MM [the United States, the EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan].
Additionally, the reports feature an examination of prominent companies working with their lead candidates in different stages of clinical development. Let’s deep dive into the market assessment of these mucopolysaccharidosis types individually.
Mucopolysaccharidosis I Market
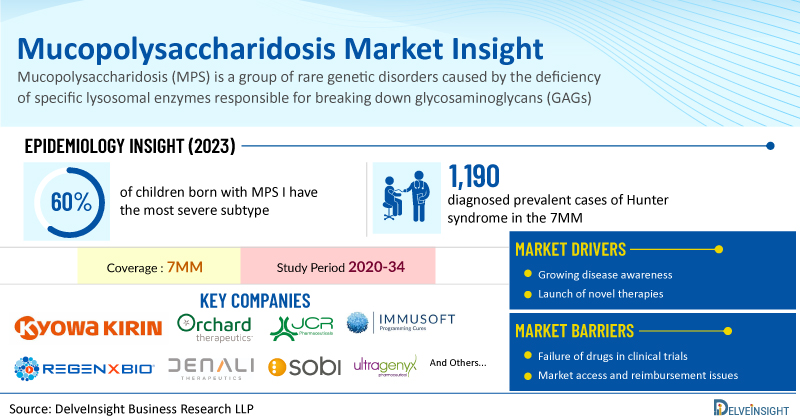
Mucopolysaccharidosis I (MPS I) is a rare genetic disorder caused by a deficiency of the enzyme α-L-iduronidase, leading to the accumulation of glycosaminoglycans (GAGs) in various tissues and organs. It is an autosomal recessive lysosomal storage disease and manifests in a spectrum of severity, ranging from the severe Hurler syndrome to the attenuated Scheie and Hurler-Scheie syndromes. Approximately 60% of children born with MPS I have the most severe subtype and rarely live past the age of 10 when untreated.
MPS I treatment primarily consists of Hematopoietic Stem Cell Transplantation (HSCT), Enzyme Replacement Therapy (ERT), or a combination of both, along with supportive care such as pain management, anti-inflammatory medications, oxygen therapy, and surgical interventions. While these treatments help alleviate symptoms and prolong survival, they cannot reverse existing damage, making early intervention essential for optimal outcomes.
Currently, ALDURAZYME (BioMarin/Sanofi) is the only FDA-approved therapy for MPS I in the US. The drug development pipeline for MPS I remains limited, with only a few emerging therapies in progress. Key companies working on new treatments include Kyowa Kirin, Orchard Therapeutics (OTL-203), JCR Pharmaceuticals (JR-171), Immusoft (ISP-001), and REGENXBIO (RGX-111). As new therapies enter clinical development and the prevalence of MPS I increases, the MPS I market is expected to expand during the forecast period (2025–2034).
For a comprehensive view of the MPS I market, check out the Mucopolysaccharidosis I Market Assessment
Mucopolysaccharidosis II Market
Hunter syndrome is a rare X-linked recessive disorder for which various initiatives are underway to ensure early diagnosis and timely treatment. Due to its low prevalence, identifying affected individuals within large populations remains challenging. Several organizations, including the MPS Society, Genes In Life, Angel’s Hand Foundation, and The Global Genes Project, are actively working to enhance the quality of life for patients and their families.
According to DelveInsight’s analysis, the total number of diagnosed prevalent cases of Hunter syndrome in the 7MM was assessed to be around 1,190 in 2023, and are projected to increase during the forecast period. Among the severity-specific cases, there were nearly 65% of severe cases of Hunter syndrome in 2023 in the US.
Current treatment options for Hunter syndrome include enzyme replacement therapy (ERT), hematopoietic stem cell transplantation (HSCT), and bone marrow transplantation (BMT). Approved medications for the condition include ELAPRASE (Takeda), HUNTERASE (GC Pharma), and IZCARGO (JCR Pharmaceuticals).
Additionally, promising emerging therapies such as DNL310 (Denali Therapeutics) and RGX-121 (REGENXBIO) are being developed to offer safer and more effective treatment alternatives. The Hunter syndrome treatment landscape is expected to undergo significant advancements between 2024 and 2034, driven by the introduction of novel therapies currently in clinical development.
Discover more about MPS II drugs in development @ Hunter Syndrome Clinical Trials
Mucopolysaccharidosis III Market
Mucopolysaccharidosis III (MPS III), also known as Sanfilippo syndrome, is a rare genetic disorder that leads to severe and fatal brain damage. It is classified as a form of childhood dementia. The condition results from a deficiency of an enzyme responsible for breaking down and recycling heparan sulfate, a complex sugar molecule. Without this enzyme, heparan sulfate accumulates in the body’s cells, particularly affecting the central nervous system. MPS III is divided into four subtypes—A, B, C, and D—each caused by mutations in different genes. While all forms lead to cognitive decline, the severity and progression rate vary depending on the specific subtype.
Currently, there are no approved treatments for MPS III. Enzyme replacement therapy has not demonstrated effectiveness for this condition, and bone marrow transplants have been attempted but yielded unfavorable outcomes. Ongoing research in MPS III is focused on gene therapy, chaperone therapy, and intrathecal enzyme therapy.
Several companies, including Allievex, Denali Therapeutics, Orchard Therapeutics, Sobi, and Ultragenyx, are developing innovative treatments. Promising drug candidates that could significantly impact the forecast period include AX 250, DNL126, UX111 (rebisufligene etisparvovec), OTL-201, and others.
In June 2024, Denali Therapeutics announced that the FDA had selected DNL126 for participation in the Support for Clinical Trials Advancing Rare Disease Therapeutics (START) Pilot Program. DNL126 is an investigational enzyme replacement therapy designed to cross the BBB for the potential treatment of MPS IIIA (Sanfilippo syndrome type A).
The ongoing research into gene therapy presents a significant opportunity for the MPS III market. As scientists develop innovative approaches to correct the underlying genetic defects associated with MPS III, there is potential for breakthrough treatments that could transform patient outcomes and expand the market.
To gain a deeper understanding of the Sanfilippo syndrome market, be sure to explore the Mucopolysaccharidosis III Market Outlook
Mucopolysaccharidosis IV Market
Mucopolysaccharidosis IV (MPS IV), also known as Morquio syndrome, is a rare autosomal recessive lysosomal storage disorder characterized by a deficiency in specific enzymes required for the degradation of glycosaminoglycans. This enzymatic deficiency leads to the accumulation of GAGs in various tissues, resulting in progressive skeletal abnormalities, restricted growth, and other systemic manifestations. Notably, intelligence typically remains unaffected in individuals with MPS IV. The onset of symptoms usually occurs between ages 1 and 3, with physical growth slowing and often ceasing around age 8. Individuals with the more severe form, MPS IVA, may have a reduced life expectancy, often not living beyond their 20s or 30s.
The global prevalence of MPS IV varies, with studies indicating a point prevalence of approximately 1 per 599,000 in the United Kingdom. Due to its rarity, the patient pool is relatively small, which poses challenges in terms of awareness, diagnosis, and the development of targeted therapies.
Current treatment strategies for MPS IV primarily focus on managing symptoms and improving quality of life. ERT has been a cornerstone of treatment, aiming to supplement the deficient enzyme and reduce GAG accumulation. However, ERT is often associated with high costs and may not be accessible to all patients, especially in developing countries. Supportive care, including surgical interventions to address skeletal abnormalities and respiratory issues, plays a crucial role in the comprehensive management of the disease.
The mucopolysaccharidosis IV treatment market has been experiencing significant growth, driven by increased research and development efforts and a focus on novel therapeutic approaches. The market’s expansion is further supported by advancements in gene therapy, improved diagnostic tools, and increased advocacy and awareness efforts, all contributing to better patient outcomes and access to treatments.
Explore in-depth for a comprehensive understanding of the Mucopolysaccharidosis IV Clinical Trials
Mucopolysaccharidosis VII Market
Mucopolysaccharidosis VII (MPS VII), also known as Sly syndrome, is a rare, inherited metabolic disorder caused by a deficiency of the enzyme β-glucuronidase, leading to the accumulation of glycosaminoglycans in tissues and organs. This accumulation causes progressive damage, primarily affecting the skeletal, cardiovascular, and nervous systems. The disorder is usually diagnosed in childhood, and symptoms may include developmental delays, coarse facial features, organomegaly, hearing loss, and joint stiffness. MPS VII has an estimated global prevalence of about 1 in 250,000 live births, with a higher incidence in certain regions due to genetic factors.
The condition is caused by mutations in the GUSB gene, which codes for β-glucuronidase. These mutations lead to reduced or absent enzyme activity, preventing the breakdown of GAGs. As a result, the body cannot clear these substances, leading to their accumulation in cells and tissues. Over time, the buildup of GAGs results in progressive organ dysfunction and severe neurological impairment. MPS VII is inherited in an autosomal recessive manner, meaning both parents must carry a copy of the mutated gene to pass it on to their offspring.
In terms of treatment, ERT has been the primary approach, where synthetic β-glucuronidase is administered to help break down the accumulated GAGs. However, ERT does not effectively address neurological symptoms, and efforts are underway to develop therapies that can penetrate the blood-brain barrier. Gene therapy and substrate reduction therapy (SRT) are emerging as promising alternatives, with clinical trials ongoing. Currently, there is no cure for MPS VII, and treatment mainly focuses on managing symptoms and improving the quality of life.
The market for MPS VII therapies is seeing growth as more companies invest in developing novel treatments. Companies like Regenxbio and Ultragenyx Pharmaceutical are pioneering gene therapy approaches, while others are advancing enzyme replacement and substrate reduction treatments. With the increasing focus on rare diseases and advancements in genetic therapies, the MPS VII treatment market is expected to grow significantly in the coming years. Additionally, as awareness of the disorder increases and diagnostic capabilities improve, the patient pool may expand, further driving demand for innovative therapies.
To access a complete analysis of the MPS VII market, visit Sly Syndrome Market Assessment
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Rare Disease Consulting Services
Delveinsight’s comprehensive rare disease consulting services encompass rare disease consulting, epidemiology-based market assessment, and primary research projects aimed at obtaining elusive data through their esteemed KOL panel.

Contact Us
Shruti Thakur
info@delveinsight.com
+14699457679
www.delveinsight.com
Distribution channels: Healthcare & Pharmaceuticals Industry, Media, Advertising & PR, Science ...
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release

